
(NASHVILLE) – Dr. Ricardo Vasquez of the Vascular Center and Vein Clinic in Bloomington, has teamed up with Wave Therapeutics, a division of woman-owned Bussert Medical, Inc. out of Nashville, Ind., to tackle the deadly and costly problem of bed sores – a medical challenge that strikes patients needing long-term care and creates tremendous liability for facilities that provide that care.

Vasquez, a vascular surgeon, has joined the company as the start-up’s chief medical officer, and will become a 15-percent equity-share partner in the business.
To date, Wave Therapeutics has received $500,000 from investors to further develop the dual-technology, break-through device that’s expected to far outperform the current best-in-class product on the market – which retails at more than $4,000.
“Wave Therapeutics has developed a real solution that has previously eluded the healthcare community,” said Vasquez. “By providing the best-in-class solution at a price point that is substantially less than competitive products, we expect to deliver significantly better outcomes to patients while also improving the bottom line for our nation’s healthcare providers.”
And bringing a better option to the market is of life-or-death urgency, says Jessica Bussert who invented the device and founded Wave Therapeutics after treating a wheel-chair bound military veteran who couldn’t afford an effective solution to prevent pressure ulcers.

“After that experience, I knew I had to do something,” said Bussert, who brings medical, engineering and technology expertise to the venture. “More than 60,000 people die in the United States each year from bed sores, which costs the U.S. healthcare industry more than $10 billion each year.”
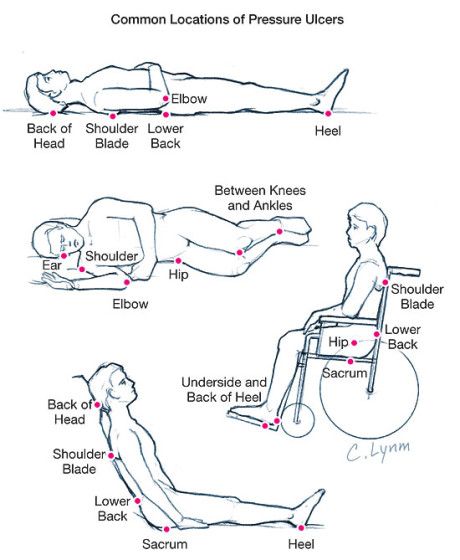
Bussert’s invention is patent-pending and has application in the medical, consumer and military markets, and has captured the attention of the U.S. Veterans Health Administration and the U.S. Department of Defense. The VHA has authorized two studies using the technology in VA hospitals across the nation.
Bussert Medical, Inc., has been valued at $3.3 million already – a significant valuation given the device is yet to complete market testing.
The startup says the current best-in-class product on the market to prevent bed sores has a retail price of more than $4,000.
“These are very expensive devices that actually have air bladders that inflate side to side and alternate the pressure points. That’s the current state-of-the-art,” said Bussert. “What Wave has done is we’ve taken that alternative pressure state-of-the-art and improved it by adding a sequential compression component to it. So not only are we eliminating those pressure hot spots, but we’re actively promoting return blood flow from the extremities back to the heart and lungs for reoxygenation.”
Bussert says she expects the retail price for the Wave device could be around $300. To help further the device’s development, the Veterans Health Administration has authorized two clinical studies of the device at VA hospitals throughout the country.
“The reason why this is so exciting is in the United States, the largest single healthcare provider there is is the Veterans Health Administration,” she added. “They’re my biggest potential customer and they’re interested enough in my product that they’re going to invest in two studies into it.”
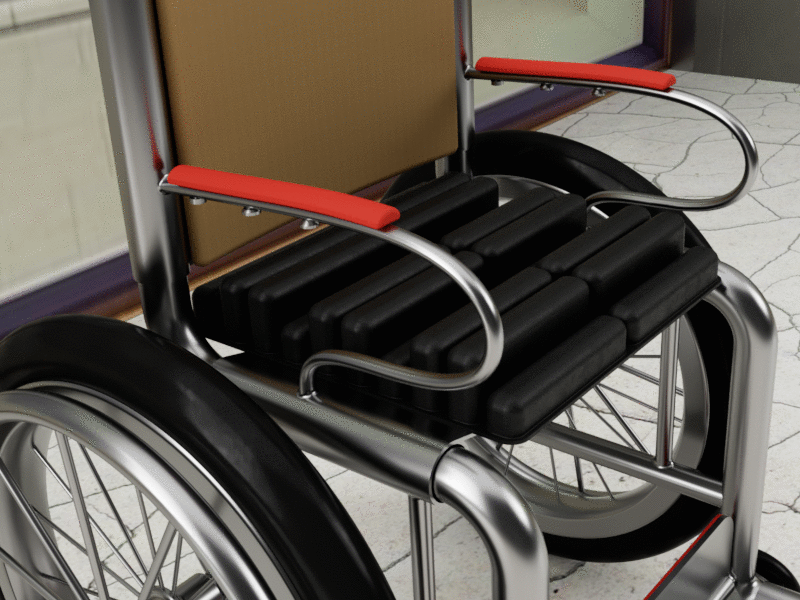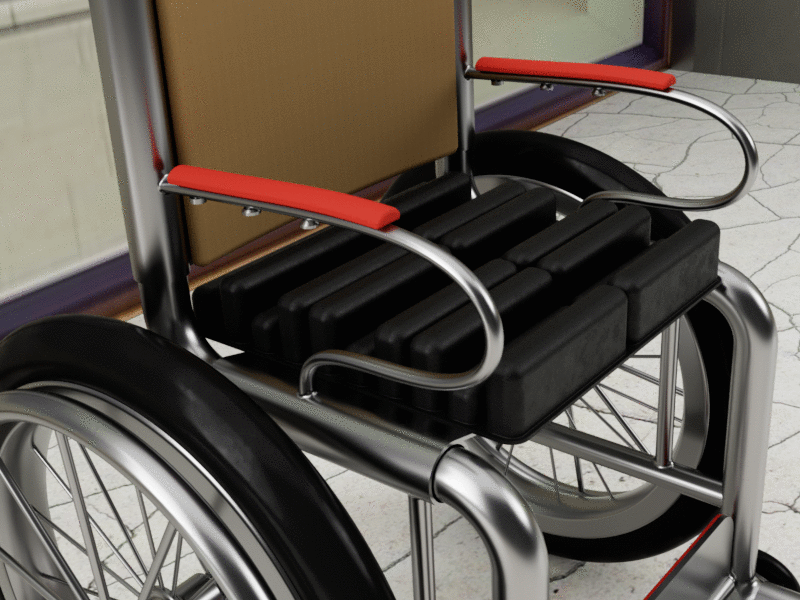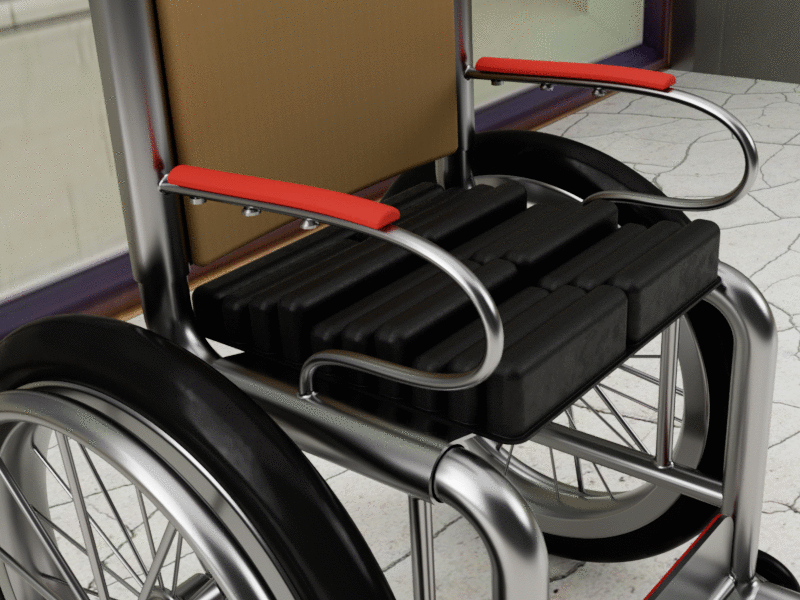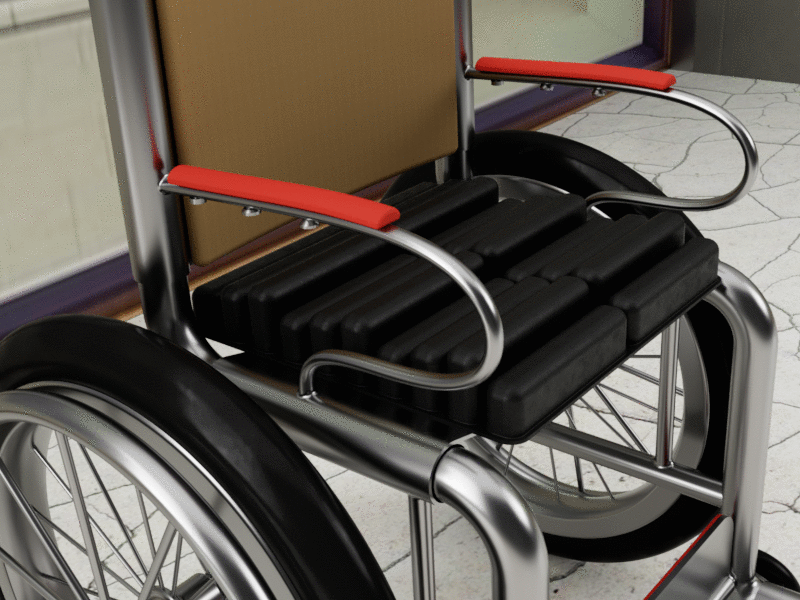
Bussert says the initial product that will include the technology will be a wheelchair cushion, but plans are in place for full mattress toppers, neo-natal beds, and stretcher mattresses. She adds the company has also identified uses for the military, as well as people who sit for extended periods of time such as frequent fliers and long-haul truck drivers.
About Wave Therapeutics
Wave Therapeutics, a division of woman-owned Busssert Medical, Inc., based in Nashville, Ind., has developed a new automated medical cushioning device designed to reduce the risks of pressure ulcers and blood clots in those that are mobility impaired due to age, health status, or occupation. Our patent-pending technology is a hybrid of two clinically tested and validated therapies combined in a novel manner, and is expected to be more effective than anything else on the market, as well as significantly less expensive than the current market leader.



