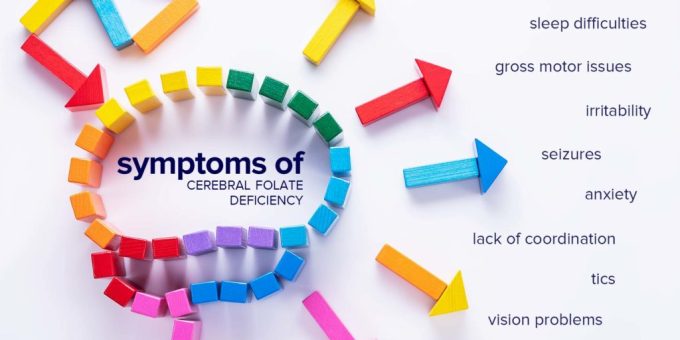
WASHINGTON D.C. – The U.S. Food and Drug Administration (FDA) has initiated the process to approve leucovorin calcium tablets for treating cerebral folate deficiency (CFD). This neurological condition impairs the transport of folate to the brain. The move is a significant step in addressing a condition that has been linked to developmental delays with autistic features, seizures, and problems with movement and coordination.
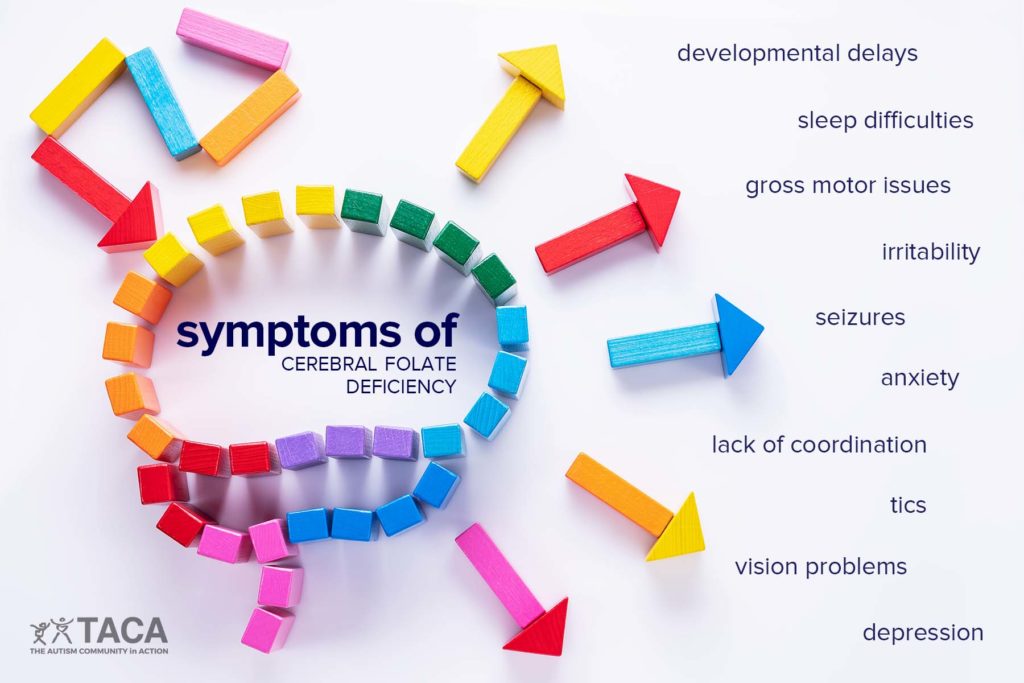
The FDA’s decision is based on a systematic analysis of scientific literature from 2009 to 2024, including case reports and other data, which demonstrate that leucovorin calcium can help individuals with CFD.
Dr. Marty Makary, the FDA Commissioner, highlighted the agency’s commitment to finding and treating the root causes of autism, noting, “We have witnessed a tragic four-fold increase in autism over two decades. Children are suffering and deserve access to potential treatments that have shown promise.”

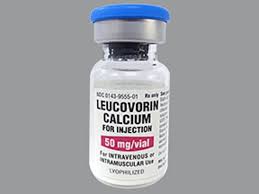
The FDA is collaborating with GSK, the manufacturer of Wellcovorin (leucovorin calcium), to update the drug’s label to include information for the safe and effective use in adults and children with CFD.

Dr. George Tidmarsh, Director of the FDA’s Center for Drug Evaluation and Research, stated, “This effort reflects the FDA’s commitment to identify opportunities to repurpose drugs to treat chronic diseases.”
While leucovorin calcium is being approved for CFD, the FDA notes that more research is needed to assess its safety and effectiveness in a broader patient population with neuropsychiatric symptoms, including autistic features. The agency remains dedicated to using “gold standard science and common sense to deliver for the American people.”



