Brought to you by WBIW News and Network Indiana
Last updated on Wednesday, May 30, 2018
(UNDATED) - A popular birth control pill is being recalled over the possibility pills are incorrectly packaged.

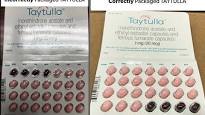
Pharmaceutical company Allergan issued a nationwide recall Tuesday for nearly 170,000 sample packs of the birth control Taytulla after a packaging error caused pills to be placed in the wrong order, a mistake that it said could lead to unintended pregnancies.
The botched packs have placebo pills where active pills should be, Allergan said in a statement. The 28-pill pack should have 24 pink pills with hormones followed by the four maroon capsules that don't have hormones, the company said. However, in the recalled packs, the placebos are at the beginning of the treatment. Taking placebos allow women to still experience period-like bleeding.
"The reversing of the order may not be apparent to either new users or previous users of the product, increasing the likelihood of taking the capsules out of order," the company's statement said. "If patients have concerns regarding the possibility of an unintended pregnancy they should consult their physician."
Missing even one day of an oral contraceptive might put women "at higher risk" of pregnancy, according to the Mayo Clinic. For birth control pills to be effective, they need to be taken every day at the same time.
The affected lot was sent to health-care providers across the United States, and the sample packs have been circulating since August, according to the statement. Allergan said it was alerted to the issue by a physician report and launched a voluntary recall. Customers are being notified of the recall by letter, and the company advised people who have the faulty packs to contact their physicians to arrange returns.
Only one lot of sample packs had misplaced pills, Allergan told STAT News.
"An investigation has been completed at the packaging site," the company said. "Given the nature of the issue, controls and inspections in place on the packaging line, the recall is limited to one lot of Taytulla. At this time, no other units with the defect have been identified within the lot or within any other Taytulla lot."
Allergan said it hasn't heard of any unintended pregnancies caused by the faulty packaging, the Wall Street Journal reported.
1340 AM WBIW welcomes comments and suggestions by calling 812.277.1340 during normal business hours or by email at comments@wbiw.com
© Ad-Venture Media, Inc. All Rights Reserved.