WEST LAFAYETTE – Rescue Biomedical has received a Fast-Track Small Business Innovation Research, or SBIR, a grant from the National Institutes of Health to develop its technology that detects when a person is overdosing on an opioid and delivers naloxone to reverse the action.
Hyowon “Hugh” Lee, Rescue Biomedical CEO, said the company looks to partner with recovery clinics and clinicians to identify and work with opioid use disorder (OUD) patients at high risk of overdosing again.

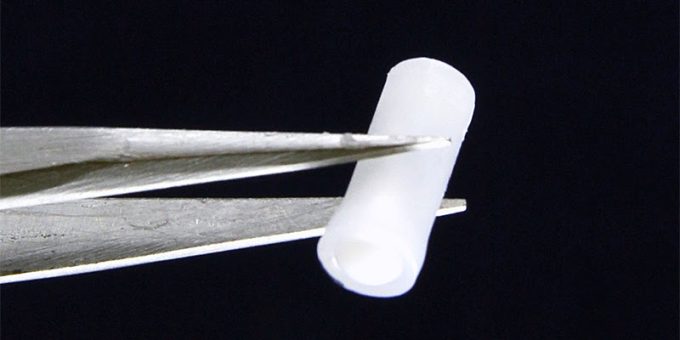
“OUD patients who recently undergo treatment are at a higher risk of accidentally overdosing again due to their lowered tolerance,” said Lee, who also is a Purdue University associate professor from the Weldon School of Biomedical Engineering, director of the Center for Implantable Devices at Purdue, and Purdue Faculty Champion in Mental Health and Substance Misuse. “Our device is a closed-loop drug delivery system that automatically detects when someone is overdosing and immediately provides life-saving naloxone to prevent long-term neurological damage or death.”
The four-year, $2.82 million grant is an expedited award. It requires administrative approval at the end of what would be a traditional Phase I period to continue to Phase II with no required additional scientific review.
Lee said Rescue Biomedical seeks to complete specific milestones through the lifetime of the grant.
“In Phase I, our goal is to better understand customer needs and identify a regulatory pathway for an approval from the U.S. Food and Drug Administration,” he said. “In Phase II, we aim to perform more usability evaluations and demonstrate functionality as we move toward regulatory approval.
“After the successful conclusion of these milestones, we will need to raise additional funds to scale up our manufacturing and to go through clinical trials to obtain regulatory approval.”
Lee said federal SBIR and Small Business Technology Transfer (STTR) grants are lifelines for small businesses like Rescue Biomedical.
“They provide us with a substantial, non-dilutive launchpad to further develop our ideas to bring to the market,” Lee said. “It gives us more visibility and credibility to other potential investors and stakeholders since we have gone through the rigors of federal review panels. We are trying to work on solving real-life problems that affect hundreds of thousands of people across the country, and the SBIR program from the NIH is enabling it.”
Rescue Biomedical’s team includes Purdue researchers Craig Goergen, the Leslie A. Geddes Associate Professor of Biomedical Engineering; Chi Hwan Lee, the Leslie A. Geddes Associate Professor of Biomedical Engineering and associate professor of mechanical engineering; and Jacqueline Linnes, the Marta E. Gross Associate Professor of Biomedical Engineering. Vy Le, a former student of Hugh Lee, also is involved with the company while also pursuing an MBA from Rice University. The company collaborates with MED Institute and Drs. Matthew Aalsma and Allyson Dir of the Indiana University School of Medicine.
Dir said medical professionals are seeing record rates of opioid overdoses. The Centers for Disease Control and Prevention reported there were 91,799 overdose deaths in the United States in 2020, an increase of 31% from 2019.
“The development of the Rescue Biomedical technology is really exciting and comes at a critical time,” Dir said. “Harm reduction is meant to save lives, and this potential technology will be a great addition to that toolkit of strategies and interventions. The more resources that are available, the more opportunities there are to improve outcomes and save lives.”
Rescue Biomedical licenses its technology through the Purdue Research Foundation Office of Technology Commercialization. It also is a client of the Purdue Foundry, an entrepreneurship and commercialization hub whose professionals help Purdue innovators create startups.



